Envirionmental Factor of Unborn Babies Seeing in Womb
Abstract
Aberrant fetal growth is associated with morbidities and mortality during childhood and adult life. Although genetic and environmental factors are known to influence in utero growth, their relative contributions over pregnancy is unknown. We estimated, across gestation, the genetic heritability, contribution of shared environment, and genetic correlations of fetal growth measures (abdominal circumference (AC), humerus length (HL), femur length (FL), and estimated fetal weight (EFW)) in a prospective cohort of dichorionic twin gestations recruited through the NICHD Fetal Growth Studies. Structural equation models were fit at the end of first trimester, during mid-gestation, late second trimester, and third trimester of pregnancy. The contribution of fetal genetics on fetal size increased with gestational age, peaking in late second trimester (AC = 53%, HL = 57%, FL = 72%, EFW = 71%; p < 0.05). In contrast, shared environment explained most of phenotypic variations in fetal growth in the first trimester (Air-conditioning = l%, HL = 54%, FL = 47%, EFW = 54%; p < 0.05), suggesting that the first trimester presents an intervention opportunity for a more than optimal early fetal growth. Genetic correlations between growth traits (range 0.34–one.00; p < 0.05) were strongest at the terminate of first trimester and declined with gestation, suggesting that different fetal growth measures are more than probable to be influenced by the same genes in early pregnancy.
Introduction
Fetal growth is an important determinant of health and disease in kid- and adult-hood. Measures of abnormality of fetal growth are associated with perinatal morbidity and mortality, and long-term adverse health outcomesane,2,3,four,5. Complex interactions betwixt genetic and environmental factors including fetal and parental genetic variations, maternal nutrition, and placental function play important roles in fetal growth6,7. Despite the cognition that size at nascence does not reflect the blueprint of fetal growth in utero, previous genetic and not-genetic studies accept primarily used birthweight as crude measure of intrauterine growth6,vii,8,nine,10,11. Studies that demonstrate genetic and not-genetic contributions to the longitudinal blueprint of growth in utero, identifying the timing when genetic and/or environmental factors during pregnancy are well-nigh influential, are lacking.
To engagement, a full of threescore loci associated with birthweight have been discovered using genome-wide association studies (GWASs)9,ten,12. About 15% of the variance in birthweight has been explained past single nucleotide polymorphisms10, reinforcing earlier findings on heritability estimates of birthweight that ranged from 25–31%13,xiv. It has previously been demonstrated that the combined outcome of seven candidate genetic loci on birthweight variance was similar to those of maternal smoking during pregnancy10, and that of 59 autosomal loci was similar to the consequence of maternal body mass alphabetize12, suggesting that genetic loci contribute considerably high variation in birthweight. Of note, five of the 7 fetal loci that were associated with birthweight, as identified past the previous GWAS reportten, were also known to influence blazon-two diabetes (ADCY5 and CDKAL1), developed blood pressure (HMGA2, ADRB1) and adult top (LCORL)10. These genes encode proteins with diverse functions including transcriptional regulation, adipogenesis, and spermatogenesis. The genes are broadly expressed in several tissues indicating multiple potential downstream furnishings in tissues (http://www.genecards.org/).
Estimates of heritability (htwo), which mensurate the proportion of total phenotypic variance attributed to additive geneticsfifteen, can be used to mensurate the extent to which fetal growth variations in a population can be explained by genetic effectssixteen. Twin studies are well suited for studying genetic and environmental influences on circuitous traits, because estimating the correlation between monozygotic (MZ) and dizygotic (DZ) twins allows measurement of the relative contributions of fetal additive genetic, shared environmental (c2) and non-shared environmental (e2) furnishings on the variance and covariance of fetal growth measures16,17.
Heritability estimates have been used to estimate the relative contributions of genetic and non-genetic factors on parameters of growth measured at nascency18,19. In addition, several studies have shown that additive genetic furnishings vary at different stages of development during infancyxx,21,22, babyhood23,24,25, adolescence and adulthood23,26,27,28. Even so, there is limited understanding of the trends in fetal genetic influences on growth trajectories in utero. Previous studies on heritability of fetal growth establish that h2 of fetal growth varies over gestation, but the studies were limited to fetal anthropometry measured in belatedly gestation and evaluated estimated fetal weight only24,29. Evidence suggests that early on life interventions can have potent effects on the cardiovascular changes that are associated with fetal growth restriction, highlighting the importance of ascertaining sensitive "window of opportunity" for intervention30. A comprehensive understanding of the fetal genetic and environmental influences on variance of a wide array of fetal growth measures volition be pivotal to understand the pathobiology of fetal growth, to serve every bit a benchmark for estimating the missing heritability of previous and future genetic studies, and to inform effective targeting of biomedical interventions. Given that fetal growth is an important determinant of health and affliction in the perinatal period31, understanding etiology of fetal growth volition have of import clinical implications30,32.
The goal of this study was to examine the relative contributions of fetal condiment genetic and ecology influences on fetal growth trajectories in a prospective accomplice of dichorionic twin gestations recruited through the NICHD Fetal Growth Studies projection. Specifically, we estimated h2, c2, and eastward2 on estimated fetal weight (EFW), intestinal circumference (Ac), humerus length (HL), and femur length (FL) at end of first trimester, mid-gestation, tardily second trimester, and tertiary trimester. Nosotros also estimated pair-wise genetic correlations between the fetal growth measures to proceeds insights on the extent to which the same genetic factor(south) influence different fetal growth measures during the progression of pregnancy.
Results
Genetic heritability of fetal growth increases throughout pregnancy
Dizygotic twins did non significantly differ from monozygotic twins with regards to their maternal and fetal characteristics and mean EFW, AC, HL and FL (Table 1). For all measures of fetal growth, h2 was highest in belatedly second trimester and lowest at the end of beginning trimester. In contrast, cii was highest at the end of first trimester and lowest in late second trimester (Fig. 1, Tabular array S1). Specifically, htwo of EFW increased from finish of first trimester (17%) to mid-gestation (41%), peaking in late second trimester (71%), and declining at calendar week 38 (66%). In contrast, c2 declined from early through late gestation: 54% at the end of offset trimester, 39% at mid-gestation, xi% at belatedly second trimester and vii% at week 38.
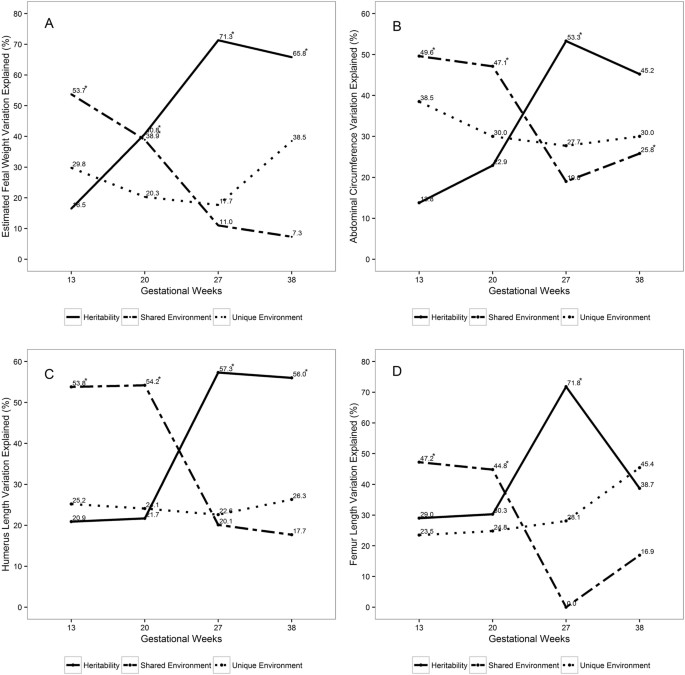
Fetal genetic heritability, shared and unique environmental variance estimates of fetal growth trajectories over gestation. (A) Estimated fetal weight (EFW). (B) Intestinal circumference (AC). (C) Humerus length (HL). (D) Femur length (FL). *Bespeak statistically pregnant estimates (P < 0.05).
For AC, h2 increased from end of showtime trimester (14%) to mid-gestation (23%), peaking in belatedly second trimester (53%), but declining at calendar week 38 (45%). In contrast, ctwo for Ac declined from end of beginning trimester (l%) to mid-gestation (47%), reaching 19% in late 2d trimester and increasing to 26% at week 38. Similar contrasting trends in h2 and c2 were observed for FL and HL. For example, h2 for FL slightly increased from first trimester (29%) to mid-gestation (30%), peaked in late second trimester (72%) and declined at week 38 (39%). c2 for FL declined from 47% at offset trimester to 45% at mid-gestation, declining to 0 at late second trimester, and rising to 17% at calendar week 38. h2 for HL continued to increase from 21% at the finish of starting time trimester to 22% in mid-gestation, and 57% in tardily second trimester, merely remained at 56% by week 38. c2 remained at 54% in starting time trimester and mid-gestation, and continued to decline to xx% at the stop of second trimester and to 18% at week 38. Overall, e2 remained relatively similar at stop of first trimester and late 2d trimester, except for HL and FL in which information technology showed an increase during the third trimester (Table S1). The corresponding p-values for htwo and ctwo estimates are shown in Table S1. Maternal age, fetal sex activity and race were covariates that were statistically significant and explained 6.1–11.1% of variance of the fetal growth measures from the end of beginning trimester to end of second trimester (Table S2).
Genetic correlation of fetal growth measures declines over gestation
Significant genetic correlations were observed between EFW and measures of skeletal growth (Table two). The genetic correlation between EFW and FL declined from first trimester (ρG = 0.79) reaching to its lowest at week 38 (ρG = 0.67). Similarly, genetic correlation between EFW and HL continually declined from the first trimester (ρG = 0.85) reaching to its everyman at week 38 (ρOne thousand = 0.65). Similar declining tendency of genetic correlations were found between Air-conditioning and FL (ρYard = 0.57 at outset trimester and ρG = 0.35 at calendar week 38), and AC and HL (ρG = 0.67 at first trimester and ρGrand = 0.39 at week 38).
Discussion
The present report estimated the heritability of fetal growth trajectories using fetal anthropometric data measured throughout gestation. To our knowledge, this is the first written report that comprehensively assessed fetal genetic and environmental influences on several longitudinal fetal growth indices and identified the timing when genetic and/or environmental factors during pregnancy are most influential. We observed substantial and increasing trends of fetal genetic influences on fetal growth across gestation, where h2 increased from first trimester to mid-gestation and peaked in belatedly second trimester. In contrast, we observed substantial decline in the contribution of ecology factors on fetal growth variation every bit gestation progresses.
A previous study establish that heritability of fetal weight decreased past 23% from week 25 to week 4229. We observed a similar pattern, where heritability of EFW decreased by ten% from week 27 to week 38. Similar to our observation, the heritability of fetal growth in the Gielen et al. study29 peaked towards tardily second trimester. Likewise, in pregnancies complicated by an abnormal glucose tolerance test, genetic factors (history of a prior large-for-gestational age newborn) appeared to predict accelerated fetal growth in the late 2d and early on third trimester (weeks 24–28)33. In contrast, some other study reported that heritability of FL and EFW increased from second trimester onwards24. The investigators in that written report indicated that their report may be prone to measurement error, leading to biased heritability estimates. In our report, the correlation between the expert reviewer and site sonographer was >88% for all growth parameters beyond visits, with 21 out of 26 measures having a correlation of ≥95%, suggesting fantabulous reliability34.
We observed that the contribution of additive fetal genetic factors to fetal growth slightly declined during the third trimester of pregnancy, whereas the variance explained past ecology factors not shared by the twin pairs showed slight increment. The third trimester is a period when the growing fetus's demand for oxygen and nutrients is loftier29. The placenta is an of import unique environment in dichorionic twins, hence a component of not-shared ecology factor with high potential to orchestrate college growth discordance between co-twins in late gestation. Placental weight, a crude marker of placental size, has been found to be independently associated with fetal growth in the third trimester35. Placenta-related factors such as differences in umbilical cord insertion sites on the placenta are also known to influence fetal growth36. Together, these information betoken that differences between dichorionic twins in factors related to placental transport functions such as placental book, placental mass, and site of umbilical cord zipper are likely to take stronger influence in fetal growth during this menstruum36,37,38, explaining our observed slight increment in the contributions of shared and unshared environmental influences and lower heritability in tardily gestation.
Shared environmental effects comprise maternal factors including historic period, nutritional status, and adiposity. Several animal and human studies demonstrated the impact of these factors at different critical pregnancy fourth dimension periods. Maternal 3rd-trimester cigarette consumption was found to be a stiff and independent predictor of birth weight percentile39. Fetuses of mothers with a higher body mass index had smaller head circumferences at early on gestation (17 weeks)forty. Maternal undernutrition and overnutrition are shown to reduce placental-fetal blood flows and stunt fetal growth in studies of animate being models41,42. In humans, maternal undernutrition in the early phase of gestation has been linked to a number of adverse furnishings on fetal growth and development43. The animal studies showed that the critical window for programing is unlike amidst the species41. In our written report, only maternal age and race every bit shared environmental factors, and fetal sex as not-shared environmental factor together explained six.1–eleven.i% of variance for each of the fetal growth measures from the cease of offset trimester to end of second trimester. Our observation that maternal age, race and infant sex together explained the phenotypic variances may suggest that futurity genome-wide association studies of fetal growth may attain ameliorate ability with models that adapted for these factors.
Our findings for genetic and environmental influences of growth for twins may not be generalizable to singletons, every bit studies reported patterns of fetal growth differ in twins and singletons34,44,45. Still, previous study by our grouping compared dichorionic twin fetuses to singletons using the current study population and found that ultrasound measured mean EFW and AC for the twins was similar to that of singletons until approximately 32 weeks46, consistent with other studies that compared singletons and twins. Get-go at 32 weeks of gestation, dichorionic twins had smaller EFW and AC compared to singletons. This observation for slower growth in twins compared to singletons could be due to lesser adequacy of sustaining adequate growth in twin fetuses throughout pregnancy47. In addition, maternal constraint, which involves a prepare of uteroplacental mechanisms by which fetal growth is restricted from reaching its genetic potential, could explicate differences in growth betwixt twins and singletons48.
The strong genetic correlations nosotros observed between different fetal growth measures particularly in early gestation indicates that skeletal growth and adipogenesis may exist modulated through a small set of genetic pathways in early pregnancy. Interestingly, we observed that genetic correlation was highest during the first trimester when the heritability of the fetal growth traits was the everyman. This will exist useful in time to come genomic studies because, if a genetic variant associated with 1 fetal growth trait in early on pregnancy is discovered, there is a high gamble that the aforementioned genetic variant also influences the other correlated traits. Consequent with our finding, a recent GWAS has demonstrated meaning genetic correlations between birthweight and birth length49. Furthermore, height and weight during infancy were plant to be strongly influenced by the same additive genetic and shared environmental factorsl.
The main forcefulness of our study its longitudinal design and implementation of a standardized ultra-sonology protocol with established quality control. Our study population included pregnancies with dichorionic twin gestations, which allowed us to assess the influence of individual environment on fetal growth (eastward.1000. placental effects). Chorionicity is associated with adverse fetal outcomes51,52,53,54. A prospective report plant worse outcomes for dichorionic twins47, while another study showed monochorionic twins had higher perinatal morbidity and bloodshed rates compared to discordant twins48. Monochorionic placentation in itself is suggested to have an inverse association with birthweight53. Future studies may benefit from evaluating both di- and mono-chorionic twins. While di-chorionic twins enable united states to study the influence of individual in-utero exposures experience by the co-twins48, mono-chorionic twin studies volition be useful to reduce confounders in studying furnishings of fetal sex and genetic differences in di-zygotic twins.
Our written report was underpowered to examine sex activity-specific genetic and environmental furnishings. Evaluating the sexual activity-specific associations is of import considering previous studies accept indicated that male person and female offspring reply differently to adverse environmental exposures55,56. Moreover, trans-generational transmission of low birthweight linking maternal birthweight to offspring birthweight has been plant to be sex activity-specific57. Information technology should be noted that variations in the relative contribution of genetic and ecology factors on fetal growth may be due to the influence of different genetic loci at unlike stages of fetal growth, different levels of influence from the aforementioned locus at unlike gestational ages, and a combination of the 2 effects likewise as cistron-environment interactions. Lastly, we have not assessed for maternal genetic effects, and factor-gene and gene-surround interaction furnishings which may farther elucidate mechanisms of fetal growth. Future genetic studies are needed to place the genetic loci and pathways underlying the longitudinal heritability changes found in the present study.
In summary, additive fetal genetics explained greater proportions of phenotypic variation in fetal growth at the stop of gestation. In contrast, shared surround explained most of phenotypic variation in fetal growth in the first trimester, suggesting that early pregnancy presents an intervention opportunity for a more than optimal early fetal growth. Our observation for contrasting trends in genetic heritability and shared environment variance for fetal growth beyond gestation suggests that environmental factors have stronger influence on growth at early on gestation, but are overtaken by genetic influences in late gestation. Our observation for strong genetic correlations between different fetal growth measures suggest that the same genes may influence skeletal growth, and fat mass in early gestation.
Methods
Written report population, setting and design
The study cohort was designed from the Eunice Kennedy Shriver National Plant of Child Health and Homo Development (NICHD) Fetal Growth Studies - twins. Briefly, a accomplice of 171 (15 MZ, 133 DZ, 8 missing with aforementioned sex activity, and 15 missing neonatal sex and zygosity) women with dichorionic twin pregnancies was recruited from viii clinical sites in U.S. betwixt 2012 and 201334,58. Twin pregnancies with confirmed zygosity determined using standard single tandem repeat identifier kits (Applied Biosystems AmpFLSTR Identifier PCR Amplification Kit; ThermoFisher Scientific, Waltham, MA) (xv MZ and 133 DZ) were included in this study. A standardized ultrasound protocol was implemented, and sonographers underwent extensive training and credentialing. Women underwent up to 7 ultrasound examinations at which the fetal anthropometric biometrics HC, AC, HL and FL were measured59. The initial ultrasound imaging was scheduled between 11 weeks 0 days and thirteen weeks 6 days of gestation. Women were them randomly assigned to receive sonograms according to schedule A (sixteen, 20, 24, 28, 32, and 35 weeks) or schedule B (18, 22, 26, 30, 34, and 36 weeks)34.EFW was calculated using the Hadlock formula, which incorporated HC, Air conditioning and FLthreescore. Zygosity of same sex twin pairs was adamant from collections of placental samples or buccal swabs using standard single tandem repeat identifier kits (Applied Biosystems AmpFLSTR Identifiler PCR Amplification Kit; ThermoFisher Scientific, Waltham, MA).
Information on sociodemographic characteristics; medical, reproductive, and pregnancy histories, and wellness and lifestyle behaviors was obtained through in person interviews conducted at each of the prenatal study visits every bit previously described34,58. The study was approved by the Institutional Review Boards of NICHD, participating clinical institutions, and data and imaging coordinating centers. Informed consent was obtained from all participants and the written report was conducted in accordance with relevant standards and guidelines.
Statistical analysis
Linear mixed models with a cubic spline mean structure and a random effects structure that included linear, quadratic, and cubic random effects, and an intercept term for the individual fetus inside twin pair61, were used to model growth trajectories for twins and ascertain anthropometric measurements at 13 weeks and 6 days (cease of first trimester), 20th week (mid-gestation), 27 weeks and half dozen days (late second trimester), and 38 weeks and 6 days of gestation (third trimester). All models included continuous variables such as maternal historic period, pre-pregnancy trunk-mass-index (BMI), and categorical variables such every bit smoking in the past half dozen months since the time of interview, alcohol use in the by week since the time of interview, race (White/non-Hispanic vs Other), parity (nulliparous vs ≥ane child), gravidity (1, 2 or ≥three pregnancies), employment status (employed vs other) educational status (≤loftier schoolhouse vs >high schoolhouse), and fetal sex (male person vs female) equally covariates. Fetal growth mensurate were inverse normalized to ensure that their residual kurtosis values were within normal range.
Twin studies allow us to estimate the contribution of condiment fetal genetic, shared ecology and non-shared environmental factors on the variance of fetal growth measuresfifteen,16. MZ twins share 100% of their genes, whereas DZ twins share 50% of their genes. Both MZ and DZ twins are assumed to exist sharing 100% of their shared environmental influences such as in utero experiences. Non-shared environmental influences, including measurement error and placenta, are assumed to exist unique to the co-twins and contribute to all differences between MZ twins.
For each fetal growth measure (i.e., EFW, Ac, HL, and FL), nosotros estimated the: (1) genetic heritability, i.e. the proportion of phenotypic variance attributed to condiment fetal genetic variancexv, (two) environmental variances (shared by both twins in a pair and unique to each co-twin), and (iii) genetic correlation betwixt fetal growth measures, which measures the proportion of covariance of ii traits explained by additive fetal genetics using the Sequential Oligogenic Linkage Analysis Routines (SOLAR) software version 7.2.five62 (http://solar-eclipse-genetics.org/). SOLAR implements a structural equation modeling approach to estimate condiment genetic heritability, shared and unique environmental contributions and the best-fitting variance component models using the maximum-likelihood method63,64,65.
Our study accomplished lxxx% statistical power to detect a 25% phenotypic variation due to condiment fetal genetics, and a 50% phenotypic variation due to shared environment at α = 0.0566 (https://genepi.qimr.edu.au//general/TwinPowerCalculator/twinpower.cgi). Prove for shared fetal genetic effects was estimated using ρGrand, where pair-wise correlations were estimated using a maximum-likelihood bivariate analysis in SOLAR. Comparing of characteristics of monozygotic and dizygotic twins was done using SAS 9.4 (SAS Institute, Cary NC).
Information availability
The datasets generated during and/or analyzed during the current study are bachelor from the NICHD Fetal Growth Studies team or the corresponding author on request, including a short protocol with a specific research question, an analysis plan, and a completed Data Use Agreement. The information, along with a set of guidelines for researchers applying for the information, will too be posted to a data-sharing site, the NICHD/DIPHR Biospecimen Repository Access and Data Sharing [https://brads.nichd.nih.gov].
References
-
Blair, Eastward. in Intrauterine growth restriction 351–366 (Springer, 2000).
-
Kessner, D. Chiliad. Infant death: an analysis by maternal hazard and health care. Vol. 1 (Plant of Medicine, 1973).
-
Marlow, N. In Intrauterine growth restriction 337–347 (Springer, 2000).
-
Osmond, C., Barker, D., Winter, P., Fall, C. & Simmonds, S. Early growth and death from cardiovascular disease in women. Bmj 307, 1519–1524 (1993).
-
Puffer, R. R. & Serrano, C. V. Patterns of birthweights (1987).
-
Sacks, D. A. Determinants of fetal growth. Current diabetes reports four, 281–287 (2004).
-
Regnault, T. R., Limesand, S. W. & Hay, W. Westward. Jr Factors influencing fetal growth. NeoReviews two, e119–e128 (2001).
-
Fradin, D., Boileau, P., Lepercq, J. & Bougneres, P. 'Non-Mendelian'genetics of fetal growth. Journal of endocrinological investigation 29, 11–15 (2005).
-
Freathy, R. One thousand. et al. Variants in ADCY5 and near CCNL1 are associated with fetal growth and nascency weight. Nature genetics 42, 430–435 (2010).
-
Horikoshi, Thousand. et al. New loci associated with birth weight identify genetic links between intrauterine growth and adult height and metabolism. Nature genetics 45, 76–82 (2013).
-
Högberg, L., Lundholm, C., Cnattingius, South., Öberg, Due south. & Iliadou, A. Birthweight discordant female twins and their offspring: is the intergenerational influence on birthweight due to genes or environment? Human Reproduction 28, 480–487 (2012).
-
Horikoshi, K. et al. Genome-broad associations for nascency weight and correlations with adult disease. Nature 538, 248–252 (2016).
-
Magnus, P., Gjessing, H., Skrondal, A. & Skjaerven, R. Paternal contribution to birth weight. Journal of Epidemiology & Community Wellness 55, 873–877 (2001).
-
Lunde, A., Melve, Yard. K., Gjessing, H. Yard., Skjærven, R. & Irgens, L. Chiliad. Genetic and environmental influences on nascence weight, nascency length, head circumference, and gestational age past use of population-based parent-offspring data. American periodical of epidemiology 165, 734–741 (2007).
-
Boomsma, D., Busjahn, A. & Peltonen, 50. Classical twin studies and beyond. Nature reviews. Genetics 3, 872 (2002).
-
Rijsdijk, F. V. & Sham, P. C. Analytic approaches to twin information using structural equation models. Briefings in bioinformatics 3, 119–133 (2002).
-
Rimfeld, K., Kovas, Y., Dale, P. S. & Plomin, R. Pleiotropy across academic subjects at the end of compulsory didactics. Scientific reports 5 (2015).
-
Clausson, B., Lichtenstein, P. & Cnattingius, Due south. Genetic influence on birthweight and gestational length determined by studies in offspring of twins. BJOG: An International Journal of Obstetrics & Gynaecology 107, 375–381 (2000).
-
Hur, Y.-M. et al. A comparison of twin birthweight information from Australia, kingdom of the netherlands, the Us, Nihon, and Republic of korea: are genetic and environmental variations in birthweight similar in Caucasians and East Asians? Twin Research and Human Genetics 8, 638–648 (2005).
-
Vlietinck, R. et al. Genetic and environmental variation in the birth weight of twins. Behavior genetics 19, 151–161 (1989).
-
Demerath, E. W. et al. Genetic and environmental influences on infant weight and weight alter: the Fels Longitudinal Study. American Journal of Human Biological science 19, 692–702 (2007).
-
Sovio, U. et al. Clan between common variation at the FTO locus and changes in body mass index from infancy to late childhood: the circuitous nature of genetic association through growth and development. PLoS genetics 7, e1001307 (2011).
-
Dubois, L. et al. Genetic and environmental contributions to weight, height, and BMI from nativity to xix years of historic period: an international study of over 12,000 twin pairs. PLOS one 7, e30153 (2012).
-
Mook-Kanamori, D. O. et al. Heritability estimates of torso size in fetal life and early childhood. PLoS I 7, e39901 (2012).
-
Silventoinen, One thousand. et al. Genetic regulation of growth in height and weight from 3 to 12 years of age: a longitudinal report of Dutch twin children. Twin Enquiry and Human Genetics 10, 354–363 (2007).
-
Jelenkovic, A. et al. Genetic and environmental influences on height from infancy to early adulthood: An individual-based pooled analysis of 45 twin cohorts. Scientific reports 6, 28496 (2016).
-
Silventoinen, Thousand. et al. Genetic regulation of growth from nativity to eighteen years of age: the Swedish young male person twins study. American Journal of Human Biology xx, 292–298 (2008).
-
Silventoinen, K. et al. Heritability of developed body height: a comparative written report of twin cohorts in eight countries. Twin Research and Man Genetics 6, 399–408 (2003).
-
Gielen, M. et al. Modeling genetic and environmental factors to increase heritability and ease the identification of candidate genes for birth weight: a twin study. Behavior genetics 38, 44–54 (2008).
-
Crispi, F., Miranda, J. & Gratacós, E. Long-term cardiovascular consequences of fetal growth restriction: biology, clinical implications, and opportunities for prevention of adult affliction. American Periodical of Obstetrics & Gynecology 218, S869–S879 (2018).
-
Barker, D. J. The origins of the developmental origins theory. Periodical of internal medicine 261, 412–417 (2007).
-
Abdul‐Karim, R. W. The clinical significance of deviations in fetal growth. International Journal of Gynecology & Obstetrics thirteen, 257–267 (1975).
-
Schaefer-Graf, U. M. et al. Determinants of fetal growth at different periods of pregnancies complicated by gestational diabetes mellitus or impaired glucose tolerance. Diabetes intendance 26, 193–198 (2003).
-
Grantz, K. L. et al. Dichorionic twin trajectories: the NICHD fetal growth studies. American periodical of obstetrics and gynecology 215, 221. e221–221. e216 (2016).
-
Roland, Thou. C. P. et al. Fetal growth versus birthweight: the office of placenta versus other determinants. PLoS i seven, e39324 (2012).
-
Loos, R. J., Derom, C., Derom, R. & Vlietinck, R. Birthweight in liveborn twins: the influence of the umbilical string insertion and fusion of placentas. BJOG: An International Journal of Obstetrics & Gynaecology 108, 943–948 (2001).
-
Kent, E. 1000. et al. Placental string insertion and birthweight discordance in twin pregnancies: results of the national prospective ESPRiT Study. American journal of obstetrics and gynecology 205, 376. e371–376. e377 (2011).
-
De Paepe, M., Shapiro, S., Young, L. & Luks, F. Placental characteristics of selective nascency weight discordance in diamniotic-monochorionic twin gestations. Placenta 31, 380–386 (2010).
-
Bernstein, I. G. et al. Maternal smoking and its association with nascence weight. Obstetrics & Gynecology 106, 986–991 (2005).
-
Wills, A. K. et al. Maternal and paternal height and BMI and patterns of fetal growth: the Pune Maternal Diet Study. Early man development 86, 535–540 (2010).
-
Vuguin, P. One thousand. Fauna models for small for gestational age and fetal programing of adult disease. Hormone Research in Paediatrics 68, 113–123 (2007).
-
Wu, Chiliad., Bazer, F. Due west., Cudd, T. A., Meininger, C. J. & Spencer, T. E. Maternal diet and fetal development. The Journal of nutrition 134, 2169–2172 (2004).
-
Coad, J., Al-Rasasi, B. & Morgan, J. Nutrient insult in early pregnancy. Proceedings of the Nutrition Society 61, 51–59 (2002).
-
Gluckman, P. D., Hanson, M. A., Cooper, C. & Thornburg, K. L. Outcome of in utero and early-life weather on adult health and disease. New England Journal of Medicine 359, 61–73 (2008).
-
Reece, Due east. A. et al. A prospective longitudinal study of growth in twin gestations compared with growth in singleton pregnancies. I The fetal head. Journal of ultrasound in medicine 10, 439–443 (1991).
-
Phillips, D. I. Twin studies in medical research: tin can they tell us whether diseases are genetically determined? The Lancet 341, 1008–1009 (1993).
-
Blickstein, I. & Keith, 50. Thousand. Neonatal mortality rates amongst growth-discordant twins, classified according to the birth weight of the smaller twin. American Journal of Obstetrics & Gynecology 190, 170–174 (2004).
-
Hanson, M. a. & Gluckman, P. Early developmental conditioning of later health and affliction: physiology or pathophysiology? Physiological reviews 94, 1027–1076 (2014).
-
Bulik-Sullivan, B. et al. An atlas of genetic correlations across human diseases and traits. Nature genetics 47, 1236–1241 (2015).
-
Van Dommelen, P., De Gunst, Thou. C., Van Der Vaart, A. W. & Boomsma, D. I. Genetic study of the pinnacle and weight process during infancy. Twin Research and Homo Genetics 7, 607–616 (2004).
-
Benson, C., Doubilet, P. & Laks, Thou. Consequence of twin gestations following sonographic demonstration of 2 middle beats in the first trimester. Ultrasound in Obstetrics & Gynecology 3, 343–345 (1993).
-
Al Riyami, N., Al-Rusheidi, A. & Al-Khabori, Thou. Perinatal outcome of monochorionic in comparison to dichorionic twin pregnancies. Oman medical journal 28, 173 (2013).
-
Papageorghiou, A., Bakoulas, V., Sebire, N. & Nicolaides, K. Intrauterine growth in multiple pregnancies in relation to fetal number, chorionicity and gestational age. Ultrasound in Obstetrics & Gynecology 32, 890–893 (2008).
-
Senoo, 1000. et al. Growth pattern of twins of unlike chorionicity evaluated by sonographic biometry. Obstetrics & Gynecology 95, 656–661 (2000).
-
Braun, J. K. et al. Bear upon of early-life bisphenol A exposure on beliefs and executive office in children. Pediatrics 128, 873–882 (2011).
-
Voigt, K., Hermanussen, K., Wittwer-Backofen, U., Fusch, C. & Hesse, Five. Sex-specific differences in nativity weight due to maternal smoking during pregnancy. European periodical of pediatrics 165, 757–761 (2006).
-
Ncube, C. N. et al. Sex-specific associations of maternal birthweight with offspring birthweight in the Omega written report. Annals of epidemiology 27, 308–314. e304 (2017).
-
Grewal, J. et al. Cohort Profile: NICHD Fetal Growth Studies–Singletons and Twins. International Journal of Epidemiology, dyx161 (2017).
-
Hediger, M. L. et al. Ultrasound Quality Assurance for Singletons in the National Institute of Kid Health and Homo Development Fetal Growth Studies. Periodical of Ultrasound in Medicine 35, 1725–1733 (2016).
-
Hadlock, F. P., Harrist, R., Sharman, R. South., Deter, R. L. & Park, S. Chiliad. Interpretation of fetal weight with the apply of caput, body, and femur measurements—a prospective written report. American periodical of obstetrics and gynecology 151, 333–337 (1985).
-
Pinheiro, J. C. & Bates, D. M. Mixed-effects models in S and S-PLUS Springer. New York (2000).
-
Almasy, L. & Blangero, J. Multipoint quantitative-trait linkage analysis in general pedigrees. The American Journal of Homo Genetics 62, 1198–1211 (1998).
-
Williams, J. T., Van Eerdewegh, P., Almasy, Fifty. & Blangero, J. Joint multipoint linkage analysis of multivariate qualitative and quantitative traits. I. Likelihood formulation and simulation results. The American Journal of Human Genetics 65, 1134–1147 (1999).
-
Kochunov, P. et al. Multi-site study of additive genetic effects on fractional anisotropy of cerebral white thing: comparison meta and megaanalytical approaches for information pooling. Neuroimage 95, 136–150 (2014).
-
Reding-Bernal, A. et al. Heritability and genetic correlation between GERD symptoms severity, metabolic syndrome, and inflammation markers in families living in Mexico Metropolis. PloS one 12, e0178815 (2017).
-
Visscher, P. M., Gordon, S. & Neale, M. C. Power of the classical twin design revisited: Two detection of common ecology variance. Twin Research and Human Genetics 11, 48–54 (2008).
Acknowledgements
This research was supported by the Intramural Inquiry Plan of the Eunice Kennedy Shriver National Found of Child Health and Human Evolution, National Institutes of Health (contract numbers: HHSN275200800013C; HHSN275200800002I; HHSN27500006; HHSN27520 0800003IC; HHSN275200800014C; HHSN275200800 012C; HHSN275200800028C; HHSN275201000009C).
Author information
Affiliations
Contributions
F.T.-A. conceived this research thought and designed the assay; J.Yard., M.B.Fifty., C.Z., and K.L.Grand. were involved in the cohort blueprint and data collection; T.W. analyzed the data; T.Due west. and F.T.-A. wrote the paper; and all authors provided critical intellectual content and approved the final manuscript.
Corresponding writer
Ethics declarations
Competing Interests
The authors declare no competing interests.
Boosted information
Publisher's notation: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary fabric
Rights and permissions
Open Access This article is licensed nether a Creative Commons Attribution 4.0 International License, which permits apply, sharing, adaptation, distribution and reproduction in any medium or format, as long as you requite appropriate credit to the original author(s) and the source, provide a link to the Creative Eatables license, and betoken if changes were fabricated. The images or other third political party cloth in this article are included in the article's Creative Eatables license, unless indicated otherwise in a credit line to the material. If material is not included in the article'due south Creative Commons license and your intended use is non permitted past statutory regulation or exceeds the permitted utilise, yous will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
Virtually this article
Cite this article
Workalemahu, T., Grantz, K.L., Grewal, J. et al. Genetic and Ecology Influences on Fetal Growth Vary during Sensitive Periods in Pregnancy. Sci Rep eight, 7274 (2018). https://doi.org/10.1038/s41598-018-25706-z
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1038/s41598-018-25706-z
Further reading
Comments
Past submitting a comment you hold to abide by our Terms and Community Guidelines. If you find something calumniating or that does non comply with our terms or guidelines please flag it as inappropriate.
Source: https://www.nature.com/articles/s41598-018-25706-z
0 Response to "Envirionmental Factor of Unborn Babies Seeing in Womb"
Post a Comment